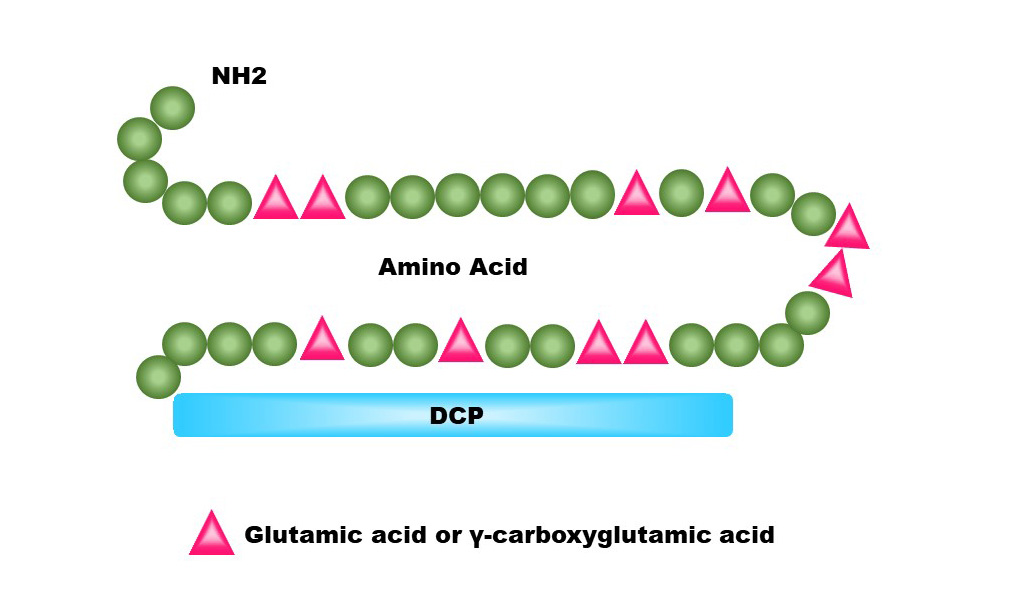
Des-gamma-carboxy prothrombin (DCP) is a serum biomarker, also known as protein induced by vitamin K absence or antagonist-II (PIVKA-II). An increase in expression of this protein indicates a higher risk for development of hepatocellular carcinoma (HCC). Adding DCP to your current surveillance practice for HCC could increase your chances of detecting early HCC.
The μTASWako DCP Immunological Test System is an in vitro device that consists of reagents used with the μTASWako i30 Immunoanalyzer to quantitatively measure, by immunochemical techniques, des-gamma-carboxyprothrombin (DCP) in human serum. The device is intended for in vitro diagnostic use as an aid in the risk assessment of patients with chronic liver disease for development of hepatocellular carcinoma (HCC) in conjunction with other laboratory findings, imaging studies, and clinical assessment.
Both DCP and AFP-L3 tests are available at major reference laboratories in the United States and are CMS reimbursed. Please contact Wako Diagnostics for more information on how to order the tests in Canada.
| CPT code | |
|---|---|
| AFP-L3 | 82107 |
| DCP | 83951 |